It’s been a busy week since my last post about Queen Fredegund. I’ve had an opportunity to shift gears and start studying the nitty-gritty of glass composition. With the permission of the Boston City Archaeologist, I borrowed 60 beads from the City Archaeology Lab. Thirty of these were discovered in the 1980s during excavations for a water line near the Charlestown Navy Yard. These beads were tossed into an abandoned cellar around 1650, as Boston’s earliest buildings were being replaced during period of rapid development. They were some of the first glass beads to arrive in North America outside of the Spanish colonies. The other thirty beads came from the Industrial School for Girls in Dorchester on Boston’s south side. They were dropped into an outhouse sometime between 1860 to 1875, and they were excavated just last year as the site was being prepared for renovations.

Although both sites fall well outside my main focus of study, this was an excellent opportunity to develop analytic techniques close to home. I took the beads to the Massachusetts Institute of Technology (MIT) Center for Materials Science and Engineering, which houses a state-of-the-art X-ray Diffraction Shared Experimental Facility. This facility is supported by the MRSEC Program of the National Science Foundation under award number DMR – 1419807, and my time there was generously supported by a grant for the public humanities being executed by Robin Fleming of Boston College. Over the course of a few days at the MIT CMSE, I used a Bruker Tracer-III with a rhodium X- ray source and a silicon-based detector to detect which elements were in the glass of each bead.
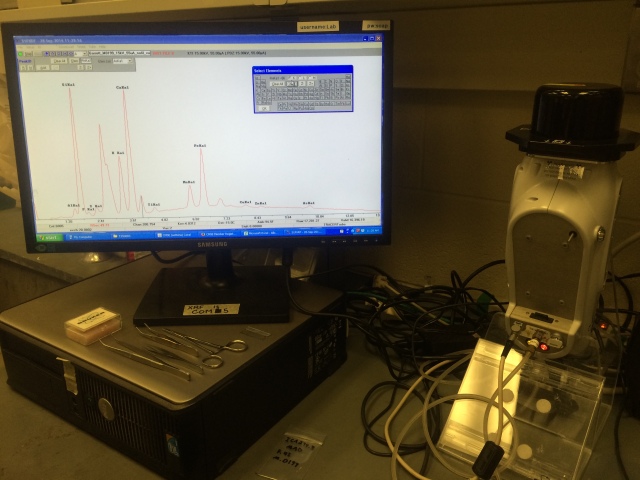
Despite the fancy engineering behind these elemental tracers, the basic science is quite simple. The Bruker tracer sends an electrical current through a Rhodium filament, just like a standard light bulb sends an electrical current through a Tungsten filament. In a regular lightbulb, the current causes electrons to get excited and jump around—a current is, after all, an imbalanced concentration of electrons that causes other electrons to jump from one atom to the next in the hopes of balancing things out. Each jump results in a release of energy that we see appear as tiny photons of light. But when an electrical current causes Rhodium electrons to jump, they produce photons at a different energy level which is invisible to the naked eye, or X-rays.
The tracer directs these photons into a stream at the given object, which in my case consisted of historic beads. The photons share their excitement with the atoms in these beads, causing the electrons to jump. When the electrons in a bead settle back down, they release energy photons of their own. The energy released by each photon depends upon how far the electron needs to jump, or just how big its atom is. Bigger atoms are heavier elements, and smaller atoms are lighter ones. The Bruker tracer measures the energy levels of the photons that come back, and so by registering just how much energy each photon has, we can tell which elements produced them. This method of analysis is known as X-ray Florescence (XRF).

For now, I’ve been looking at qualitative data. For example, the chart above shows the readings from seven black beads excavated at the Industrial School for Girls. Six of them have the same peaks and valleys, but the green line has a different quality. It’s surprising that the green line is flat at the energy levels characteristic for Silicon (Si), a base element for glass, and Manganese (Mn), an element often used to blacken glass. The green line, however, shows unexpected peaks at Sulfur (S) and Titanium (Ti). So even without knowing the exact proportions of the elements that may have produced these peaks and valleys, we can tell that the green line represents a bead that isn’t glass.
In fact, the green line belongs to a bead probably made of vulcanized rubber, which was still a relatively recent invention when this bead fell into a privy in the 1860s. In 1844, Charles Goodyear had secured the patent, claiming: “My principal improvement consists in the combining of sulphur and white lead with the india-rubber.” India rubber referred to tree rubbers that were then being cultivated in both Asia and South America, and factories were soon using Sulfur to vulcanize this rubber in both Europe and North America. The Sulfur peak indicates that the key hardening agent is present in this sample, although a conspicuous lack of lead shows that recipes had already changed.
A bead like this one adapted the hard and dark qualities of this new rubber recipe to imitate the more costly jet, which was a mineral popularly used for jewelry in the 1800s. We know that Whitby in England was then the major center for Victorian jet production, whereas glass came predominantly from Bohemia (in the modern Czech Republic) or Venice, Italy. With all these places in mind, this graph helps us see how during the 1800s even the poorest Americans—girls who had been sent away to a school for wayward youth during those inward-looking decades of the Civil War and Reconstruction—had material contacts with a very international consumer culture. Is globalization that new after all?

My next step is quantification, that is, taking these energy readings and deducing the likely glass recipes that would have produced them. I’m much indebted to Lee Drake for introducing me to this possibility. This type of analysis has the potential to be especially important for the early beads from Charlestown. All my Charlestown beads are turquoise, but the recipes for turquoise beads changed during the colonial period. These changes are already an active area of study for scholars like Ron Hancock and Elliot Blair. Quantifying my data will help me compare it to their earlier studies. These comparisons might help us more precisely date exactly when these beads were buried.
Remarkably, although the original excavators thought this filled-in cellar was “one of the earliest and best preserved colonial domestic artifact assemblages discovered archaeologically in New England,” we don’t actually know when these artifacts were buried. Our best guess is 1640–1660, based on the styles of a handful of smoking pipes found in the mixed assemblages. The ceramics (pottery) found in the cellar artifacts could date from this period, but ceramics had long lifespans and don’t actually help us narrow down a precise date. Beads, in contrast, had very short shelf-lives, so they might very well be our best opportunity for better understanding when exactly this assemblage of the neighbors’ trash was thrown together into an empty Charlestown cellar. This, in fact, is not so different from the short life-spans of popularity enjoyed by early medieval beads. Perhaps these sites aren’t so different from my own after all.

